
Evidence-based diagnostics built for
Evidence-based diagnostics built for
modern care
clinical innovation
better outcomes
modern care
modern care
clinical innovation
better outcomes
modern care
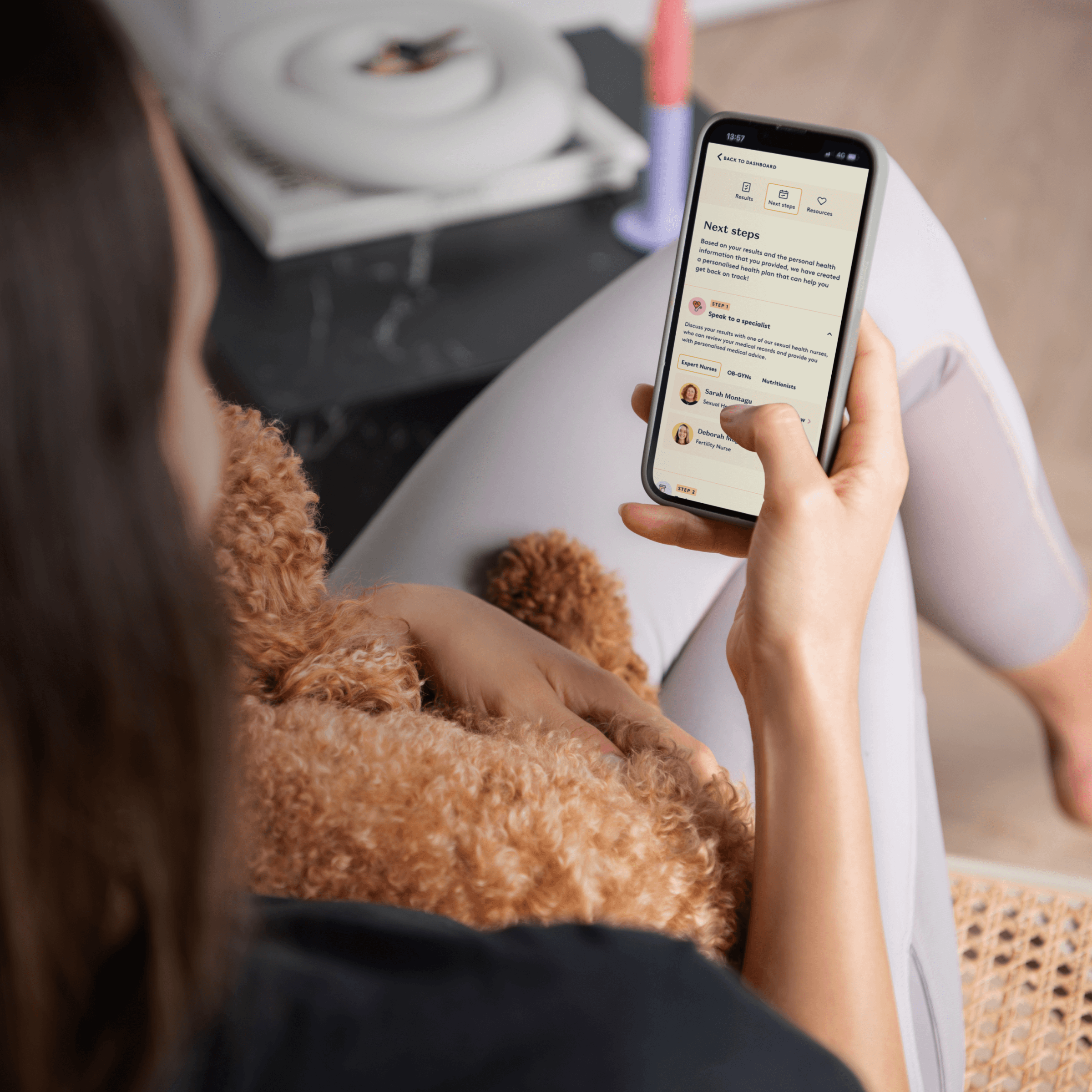
Daye’s diagnostic platform brings clinician-level accuracy to at-home testing—improving uptake, reducing barriers, and supporting informed clinical decisions.



Working with
Working with
Working with
Working with
Why partner with Daye?
Why partner with Daye?
Improved patient adherence
Self-sampling significantly increases completion rates, including among patients who avoid speculum exams or phlebotomy, resulting in more reliable follow-through.
Clinically validated diagnostics
High-yield tampon samples support accurate HPV, STI, and microbiome PCR testing from a single specimen. Venous-validated capillary devices provide reliable at-home hormone measurement.
Exceptional operational
efficiency
Reduce in-clinic workload by shifting sampling out of the exam room while maintaining diagnostic accuracy and lowering invalid/insufficient samples.
How it works
Seamless integration with clinical partners
Seamless integration with clinical partners
Clinician orders the test via API; kit shipped directly to the patient.
Clinician orders the test via API; kit shipped directly to the patient.
Clinician orders the test via API; kit shipped directly to the patient.
Patient self-collects using the validated sampling device and sends the sample to the lab.
Patient self-collects using the validated sampling device and sends the sample to the lab.
Patient self-collects using the validated sampling device and sends the sample to the lab.
Accredited labs run regulated assays; results returned in 3–5 days.
Accredited labs run regulated assays; results returned in 3–5 days.
Accredited labs run regulated assays; results returned in 3–5 days.
Clinician-guided treatment or follow-up available where clinically indicated.
Clinician-guided treatment or follow-up available where clinically indicated.
Clinician-guided treatment or follow-up available where clinically indicated.

Our diagnostic platform
Clinically validated, globally trusted diagnostics
Clinically validated, globally trusted diagnostics
Diagnostic Tampon™
Diagnostic Tampon™
High-yield, clinically-validated tampon sampling for HPV, STI, and microbiome testing.



Daye Hormone Test
Daye Hormone Test
Efficient at-home sampling, enabling fast, reliable hormone testing through accredited labs.



Trusted by providers for clinical accuracy and better patient outcomes.
Trusted by providers for clinical accuracy and better patient outcomes.
“The Daye Diagnostic Tampon has significantly enhanced our ability to provide comprehensive gynecological care. Its simple, at-home screening process empowers our patients to better understand their vaginal health symptoms, such as discharge, odor, inflammation, and pain. This level of accessibility and ease of use has been instrumental in encouraging more women and AFAB individuals to take proactive steps in managing their sexual health.
Working with the Daye team has been an absolute pleasure. Their commitment to raising the standards in gynecological care aligns perfectly with Wisp’s mission to increase access to vaginal healthcare. The partnership has allowed us to streamline diagnostics and care, creating a seamless experience for our patients from screening to treatment.”
“The Daye Diagnostic Tampon has significantly enhanced our ability to provide comprehensive gynecological care. Its simple, at-home screening process empowers our patients to better understand their vaginal health symptoms, such as discharge, odor, inflammation, and pain. This level of accessibility and ease of use has been instrumental in encouraging more women and AFAB individuals to take proactive steps in managing their sexual health.

Monica Cepak
CEO of Wisp
Our accreditation
Our accreditation
Our research is backed and guided by top-rated and accredited research bodies, dedicated to evidence-based science.

FDA audited facility



Published research
Efficacy and acceptability of a self-collected medical grade tampon as a novel vaginal sample collection tool for the detection of HPV and STIs
Center for Applied Scince and Innovation
This study aims to compare the efficacy and suitability of a self-collected tampon for the detection of human papillomavirus (HPV) and sexually transmitted infections (STIs) using qualitative TMA-based assays
Efficacy and acceptability of a self-collected medical grade tampon as a novel vaginal sample collection tool for the detection of HPV and STIs
Center for Applied Scince and Innovation
Efficacy and acceptability of a self-collected medical grade tampon as a novel vaginal sample collection tool for the detection of HPV and STIs
Center for Applied Scince and Innovation
This study aims to compare the efficacy and suitability of a self-collected tampon for the detection of human papillomavirus (HPV) and sexually transmitted infections (STIs) using qualitative TMA-based assays
Can samples taken with Daye tampons be used after being freezed?
University of Liverpool
There’s no statistically significant difference between corresponding fresh and frozen samples, enabling the conclusion that samples can be frozen before processing. The abbility to freeze tampon samples allows for processing at a more practical time.
Can samples taken with Daye tampons be used after being freezed?
University of Liverpool
Can samples taken with Daye tampons be used after being freezed?
University of Liverpool
There’s no statistically significant difference between corresponding fresh and frozen samples, enabling the conclusion that samples can be frozen before processing. The abbility to freeze tampon samples allows for processing at a more practical time.
Menstrual Tampons Are Reliable and Acceptable Tools to Self-Collect Vaginal Microbiome Samples
Center for Applied Scince and Innovation
Menstrual tampons are a more effective tool for sample collection compared to the vaginal and cervical swabs. There are confirmed similar results in literature with reports of high specificity with variable sensitivity.
Menstrual Tampons Are Reliable and Acceptable Tools to Self-Collect Vaginal Microbiome Samples
Center for Applied Scince and Innovation
Menstrual Tampons Are Reliable and Acceptable Tools to Self-Collect Vaginal Microbiome Samples
Center for Applied Scince and Innovation
Menstrual tampons are a more effective tool for sample collection compared to the vaginal and cervical swabs. There are confirmed similar results in literature with reports of high specificity with variable sensitivity.
Vaginal tampons as specimen collection device for the molecular diagnosis of non-ulcerative sexually transmitted infections in antenatal clinic attendees
2004 • P D J Sturm, Cathy Connolly, Nasreen Khan, Safiya Ebrahim, A Willem Sturm
Cervical and vaginal swabs, tampons and urines were collected from 185 first-time antenatal clinic attendees. Cultures and nucleic acid amplification assays (NAA) were performed. The sensitivity of PCR on tampons for Trichomonas vaginalis was 94% (CI 85-98%) significantly higher (P < 0.001) than culture (50%, CI 38-62%) or urine (53%, CI 41-65%).
Vaginal tampons as specimen collection device for the molecular diagnosis of non-ulcerative sexually transmitted infections in antenatal clinic attendees
2004 • P D J Sturm, Cathy Connolly, Nasreen Khan, Safiya Ebrahim, A Willem Sturm
Vaginal tampons as specimen collection device for the molecular diagnosis of non-ulcerative sexually transmitted infections in antenatal clinic attendees
2004 • P D J Sturm, Cathy Connolly, Nasreen Khan, Safiya Ebrahim, A Willem Sturm
Cervical and vaginal swabs, tampons and urines were collected from 185 first-time antenatal clinic attendees. Cultures and nucleic acid amplification assays (NAA) were performed. The sensitivity of PCR on tampons for Trichomonas vaginalis was 94% (CI 85-98%) significantly higher (P < 0.001) than culture (50%, CI 38-62%) or urine (53%, CI 41-65%).

© 2025 Anne's Day Inc 2140 S.Dupont Highway, Camden, Delaware, 19934
Terms & Conditions
Privacy Policy
Cookie Policy
For Clinicians
Solutions
Research
About us

FDA audited facility




Daye tampons are manufactured in accordance with medical device standards, including ISO13485 and GMP. The Vaginal Microbiome Testing Kit is intended for general wellness purposes only and is not intended to diagnose, treat, cure, or prevent any disease. Test results provide information about your vaginal microbiome and should not be used to make medical decisions. Any mention of specific conditions or medical terms is for educational purposes only and does not constitute a medical diagnosis.

© 2025 Anne's Day Inc 2140 S.Dupont Highway, Camden, Delaware, 19934
Terms & Conditions
Privacy Policy
Cookie Policy
For Clinicians
Solutions
Research
About us

FDA audited facility




Daye tampons are manufactured in accordance with medical device standards, including ISO13485 and GMP. The Vaginal Microbiome Testing Kit is intended for general wellness purposes only and is not intended to diagnose, treat, cure, or prevent any disease. Test results provide information about your vaginal microbiome and should not be used to make medical decisions. Any mention of specific conditions or medical terms is for educational purposes only and does not constitute a medical diagnosis.

© 2025 Anne's Day Inc 2140 S.Dupont Highway, Camden, Delaware, 19934
Terms & Conditions
Privacy Policy
Cookie Policy
Daye tampons are manufactured in accordance with medical device standards, including ISO13485 and GMP. The Vaginal Microbiome Testing Kit is intended for general wellness purposes only and is not intended to diagnose, treat, cure, or prevent any disease. Test results provide information about your vaginal microbiome and should not be used to make medical decisions. Any mention of specific conditions or medical terms is for educational purposes only and does not constitute a medical diagnosis.